Abstract
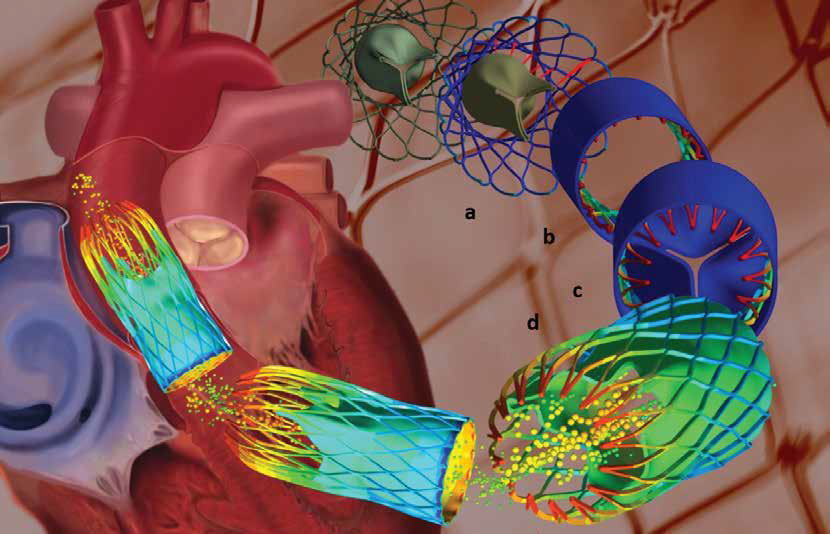
Aortic valve stenosis - a narrowing of the aortic valve - affects about 2% of adults over the age of 65. It is a chronic progressive disease whose symptoms include chest pain, difficulty breathing and fainting, and in some cases congestive heart failure if the valve is not replaced. For the past 40 years, surgeons have used open-heart surgery to replace the aortic valve. This procedure has a 2% operative mortality rate for low-risk patients younger than 70 years old, but a 24% mortality rate for high-risk elderly patients over the age of 90. As a result, cardiologists have been considering less-invasive approaches, one of which is transcatheter aortic valve replacement or implantation. Via an entry-point in a groin artery, a catheter delivers an expandable stent to the heart where it is expanded against the aortic wall to hold the existing valve open and secure the replacement valve in its place. Since this technique has only been performed a small number of times, surgeons still have many questions about the stent’s efficiency and resistance over time, amongst others. Since this cannot be accurately measured in an implanted stent, manufacturers decided to use Multiphysics to simulate the process to better understand the method and to calculate the forces operating on the implant in order to improve the stent design and the surgical procedure, as described in this article.
Read the article